Everything you need to know about the MTHFR mutation and the desire to have children
- Almost every second woman in Europe carries an MTHFR mutation - often without knowing it.
- Those affected cannot optimally utilize conventional folic acid - there is a risk of functional folate deficiency.
- The mutation can lead to increased homocysteine levels, which increases the risk of thrombosis and miscarriage.
- A lack of bioactive folate can make it difficult for the embryo to implant in the uterine lining.
- In addition to bioavailable folate, vitamin B12, B6 and omega-3 fatty acids are also important.
MTHFR mutation: the silent cause of miscarriages and implantation problems
The MTHFR mutation is a widespread genetic phenomenon that affects many women and couples who wish to have children. This genetic mutation affects an important metabolic pathway and can impair both fertility and embryonic development.
In this comprehensive guide, you will learn all about the effects of this genetic variant on pregnancy and the desire to have children, as well as effective treatment approaches.
What is an MTHFR mutation?
The MTHFR mutation affects an enzyme called methylenetetrahydrofolate reductase. This enzyme is responsible for converting folic acid into the bioactive form of folate that your body can actually use.
If you have an MTHFR mutation, this "key" does not work properly. This means that your body cannot optimally convert normal folic acid into bioavailable folate. This can lead to a functional folate deficiency, even if you consume sufficient folic acid.
How common is the MTHFR mutation?
The MTHFR mutation is surprisingly common. Around 30-40% of women in Europe have the heterozygous form (one copy of the altered gene), while 9-11% have the homozygous MTHFR mutation. This means that almost every second woman is affected to some degree (van der Put, 1995).
Link to other coagulation disorders
The MTHFR mutation is often not present on its own, but together with other genetic factors. It is found particularly frequently in combination with factor V Leiden or the prothrombin mutation 20210G>A. These combinations can further increase the risk of thrombosis and pregnancy complications (Park, 2014).
Effects on the desire to have children and pregnancy
Challenges getting pregnant
The MTHFR mutation can affect your desire to have children in various ways:
- Impairment of ovarian function: activated folate is crucial for embryonic development and ovarian function. Research shows that women with MTHFR mutations can have significantly reduced estradiol production (Sanghavi, 2024). This can impair egg maturation and reduce the chances of natural conception.
- Problems with implantation: Folate is also important for the implantation of the embryo in the uterine lining. A lack of activated folate can result in fertilized eggs not being able to implant properly in the uterus.
- Elevated homocysteine levels: The MTHFR mutation leads to elevated homocysteine levels in the blood. This increases the risk of thrombosis and other cardiovascular diseases, which can have a negative impact on fertility (Murphy, 2000).
Risk of miscarriage and habitual abortions
The issue of habitual abortions is particularly stressful for couples who wish to have children. Habitual miscarriages - defined as three or more consecutive miscarriages - can occur in up to 1-2% of all pregnancies. The MTHFR mutation can increase the risk of these repeated pregnancy losses.
Why do miscarriages occur?
- Thrombosis tendency: Elevated homocysteine levels can also lead to an increased tendency to thrombosis in the placenta
- Developmental disorders: A lack of activated folate can impair early embryonic development
- Risk of intrauterine fetal death: In severe cases, intrauterine fetal death can even occur, especially in the second half of pregnancy (Tiwari, 2015)
Spina bifida and neural tube defects
One of the most serious complications of the MTHFR mutation is the increased risk of spina bifida and other neural tube defects. Studies show that homozygous carriers of the C677T mutation have a 70% increased risk of spina bifida (de Franchis, 2002). These defects develop between day 22 and 28 of pregnancy, often before a woman even knows she is pregnant (Yan, 2012).
Diagnosis and tests
When should you get tested?
MTHFR testing may be useful as part of a basal coagulation status if:
- You have been trying to get pregnant for a long time
- You have already experienced habitual abortions
- Elevated homocysteine levels have been measured in the blood
- There is a family history of pregnancy complications
- There are other coagulation disorders such as factor V Leiden
What tests are available?
- Genetic testing: The MTHFR mutation is detected by a simple blood test as part of the basal coagulation status. The test identifies the two most common variants (C677T and A1298C) and can be performed simultaneously with tests for factor V Leiden and the prothrombin mutation 20210G>A.
- Homocysteine measurement: Elevated homocysteine levels can be an indicator of an MTHFR mutation. Values above 12 µmol/l are already considered elevated and indicate a folic acid deficiency.
- Folate status: Measuring the folate level in the red blood cells provides information about your actual folate status.
The right folic acid supplementation
Why not all folic acid is the same
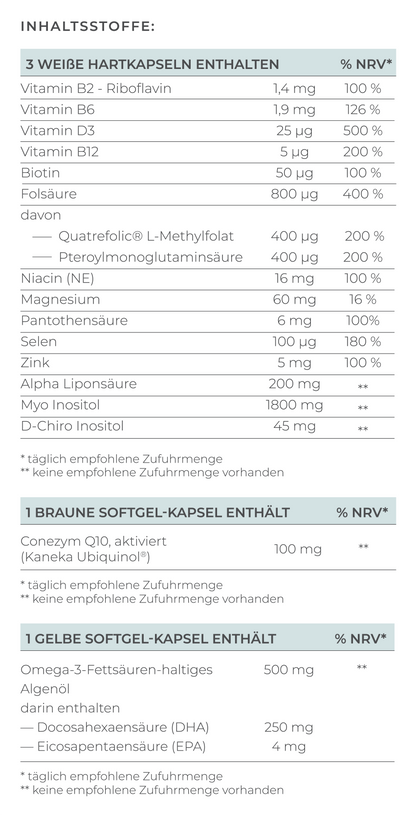
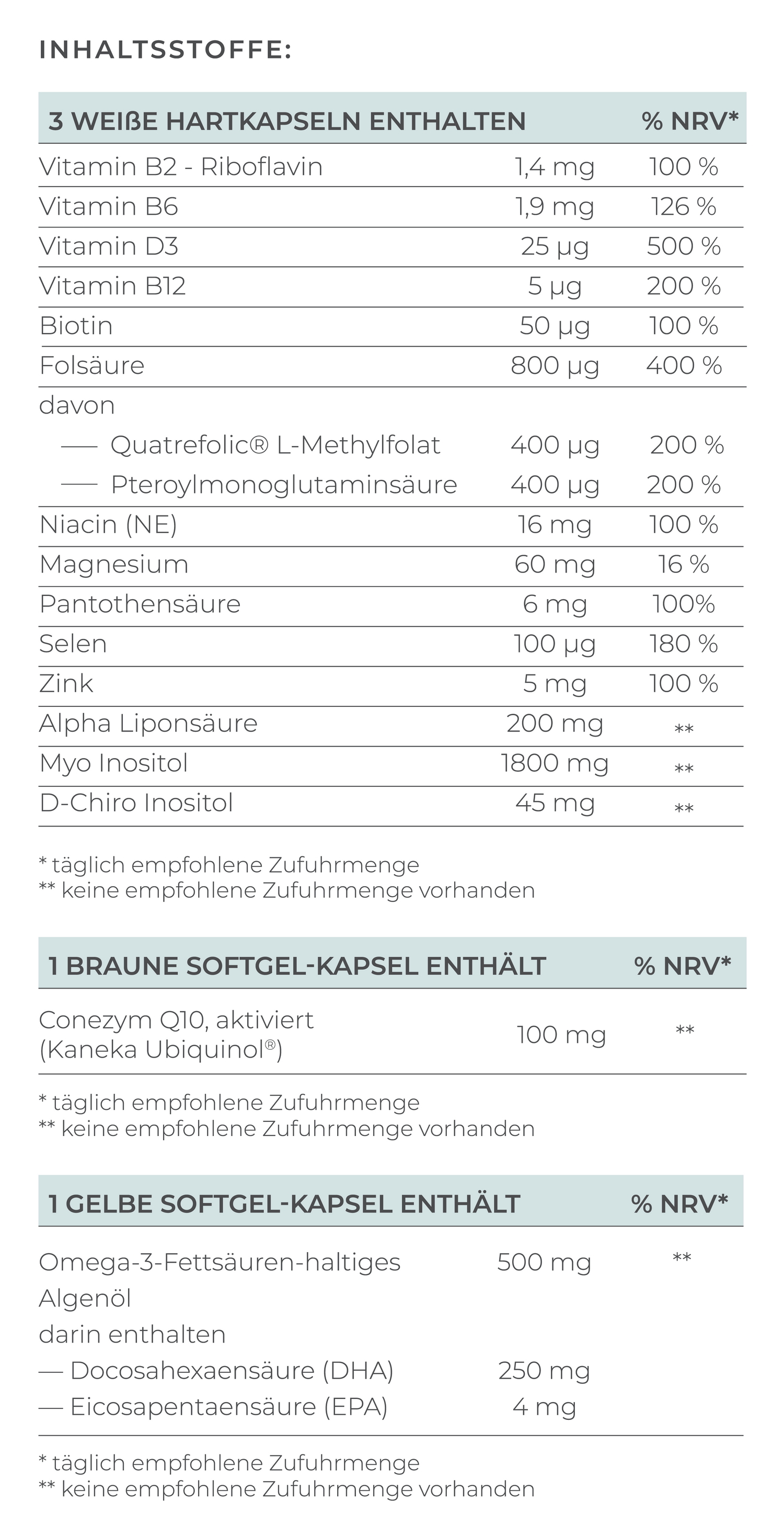
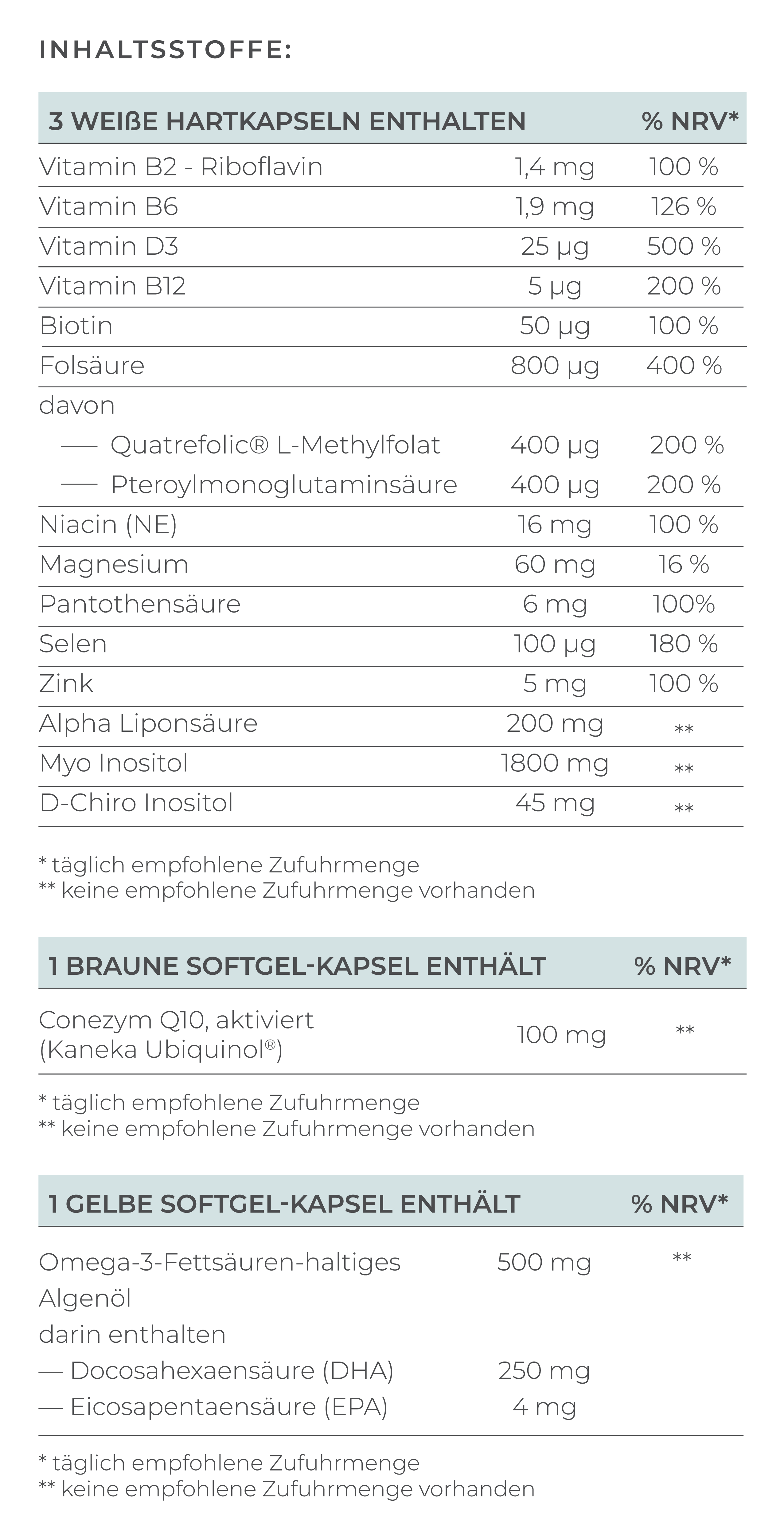
The most important element of treatment is supplementation with the right form of folate. Women with MTHFR mutations cannot convert conventional folic acid into the bioactive form of folate. Therefore, it is crucial to use bioavailable folate.
The bioactive form vs. conventional folic acid
- Bioavailable folate (L-methylfolate): Can be used directly by the body without conversion
- Conventional folic acid: Must first be converted, which is problematic in the case of MTHFR mutation
Specially developed preparations for women with MTHFR mutation
There are high-quality preparations with bioavailable folate in the form of Quatrefolic® - a patented form of L-methylfolate, such as VILAVIT Female, which help the body to absorb folate particularly well and are specially developed for patients with MTHFR mutation.
Dosage and recommended intake
Preconception advice - The right preparation:
- Start taking at least 3 months before planned conception
- 400-800 μg bioactive folate daily at normal risk
- In special cases, the doctor may also prescribe a higher dose of folic acid.
During pregnancy:
- Continuous intake throughout pregnancy
- Regular checks of folate and homocysteine levels
Comprehensive nutrient supply with B complex
In addition to folate, other B vitamins are important, which is why a B complex is often recommended:
- Vitamin B12: Works closely with folate and is important for the breakdown of homocysteine. A vitamin B12 deficiency can exacerbate the problems of the MTHFR mutation
- Vitamin B6: Supports hormone activity and folate metabolism, can also help with morning sickness
- B complex preparations: Contain all important B vitamins in balanced doses and support the entire metabolism
Additional therapeutic approaches
In certain cases - such as repeated miscarriages or an increased risk of pregnancy complications - the specialist treating you may consider additional measures. These include, for example, anticoagulant medication, low-dose platelet aggregation inhibitors or a higher dose of folic acid.
These treatments are individually adapted and only carried out under medical supervision in specialized fertility centers.
Conclusion
An MTHFR mutation is nothing to worry about - but it is an important indication that your body may need targeted support. It can affect fertility, increase the risk of miscarriage and lead to developmental disorders in the embryo, especially if there are also elevated homocysteine levels or other coagulation disorders.
In such cases, it can be useful to ensure a targeted supply of nutrients with bioavailable folate, omega-3 fatty acids, coenzyme Q10, antioxidants and other B vitamins.
High-quality micronutrient supplements containing these active ingredients can help to optimally support the metabolism - especially during the fertile phase and early pregnancy.
Please discuss your individual intake with your doctor or an experienced fertility specialist.
References
de Franchis et al. Spina bifida and folate-related genes: A study of gene-gene interactions. Genetics in Medicine (2002).